Engineering Software
Carnot Cycle Analysis -- Free One (1) Credit Hour of CE
Carnot Cycle
Introduction
This section provides a Carnot Cycle analysis when the working fluid is air.
Analysis
In the presented Carnot Cycle analysis, only air is considered as the working fluid behaving as a perfect gas -- specific heat has a constant value. Ideal gas state equation is valid -- pv = RT.
Air enters a compressor at point 1 and it exits the compressor at point 2. Isentropic compression is considered with no entropy change. Air enters a heat exchanger -- heat addition -- at point 2 and it exits the heat exchanger at point 3. At a constant temperature, heat addition takes place. Air enters a turbine at point 3 and it exits the turbine at point 4. Isentropic expansion is considered with no entropy change. Air enters a heat exchanger -- heat rejection -- at point 4 and it exits the heat exchanger at point 1. At a constant temperature, heat rejection takes place. It should be mentioned that air at point 1 enters the compressor and the cycle is repeated.
Figure 1 contains a Carnot Cycle schematic layout.
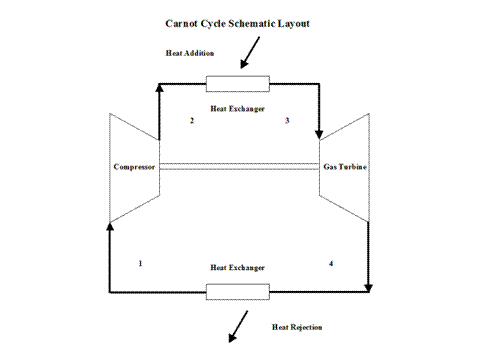
Figure 1 - Carnot Cycle Schematic Layout
Figure 2 presents a Carnot Cycle entropy vs temperature diagram.
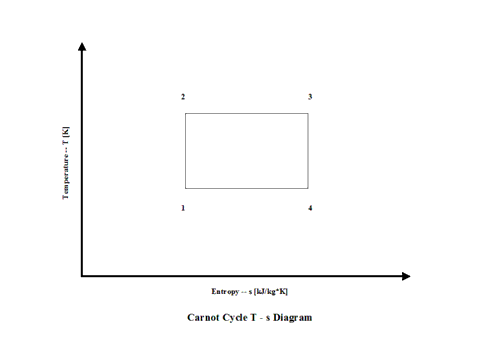
Figure 2 - Carnot Cycle Temperature vs Entropy Diagram
Figure 3 presents the Carnot Cycle efficiency as a function of the heat addition temperature. It should be noted that the inlet conditions are standard ambient conditions: temperature of 298 [K] and absolute pressure of 1 [atm].
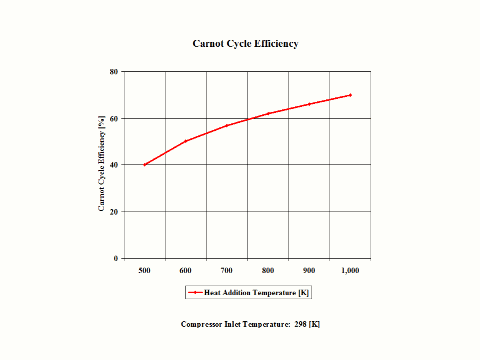
Figure 3 - Carnot Cycle Efficiency vs Heat Addition Temperature
Figure 4 presents the Carnot Cycle efficiency as a function of the heat rejection temperature. It should be noted that the turbine inlet temperature is at 800 [K].
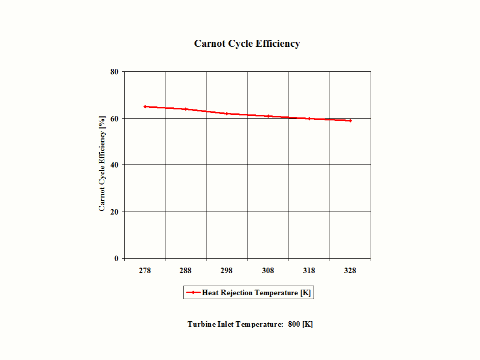
Figure 4 - Carnot Cycle Efficiency vs Heat Rejection Temperature
One can notice that the Carnot Cycle efficiency increases with an increase in the heat addition temperature when the heat rejection temperature does not change at all. One can notice that the Carnot Cycle efficiency decreases with an increase in the heat rejection temperature when the heat addition temperature does not change at all.
Assumptions
Working fluid is air. There is no friction. Compression and expansion are isentropic -- there is no entropy change. During heat addition and heat rejection, the air temperature does not change. Ideal gas state equation is valid -- pv = RT. Air behaves as a perfect gas -- specific heat has a constant value.
Governing Equations
T2/T1 = (p2/p1)^(ϰ-1)/ϰ
T3/T4 = (p3/p4)^(ϰ-1)/ϰ
ϰ = cp/cv
cp - cv = R
pv = RT
efficiency = 1 - T1/T2
efficiency = 1 - TR/TA
Input Data
T1 = 298 [K]
p1 = 1 [atm]
R = 0.2867 [kJ/kg*K]
cp = 1.004 [kJ/kg*K]
ϰ = 1.4 [/]
Results
Carnot Cycle Efficiency vs Heat Addition Temperature
Heat Rejection Temperature = 298 [K]
Heat Addition
Temperature
[K]
500
600
700
800
900
1,000
Carnot Cycle
Efficiency
[%]
40
50
57
62
66
70
Carnot Cycle Efficiency vs Heat Rejection Temperature
Heat Addition Temperature = 800 [K]
Heat Rejection
Temperature
[K]
278
288
298
308
318
328
Carnot Cycle
Efficiency
[%]
65
64
62
61
60
59
Conclusions
The Carnot Cycle efficiency increases with an increase in the heat addition temperature when the heat rejection temperature does not change at all. Furthermore, the Carnot Cycle efficiency decreases with an increase in the heat rejection temperature when the heat addition temperature does not change at all. The Carnot Cycle efficiency is not dependent on the working fluid properties.
References
JANAF Thermochemical Data - Tables, 1970